02 Understanding Cell Therapy
Section 1
What is Cell Therapy?
Learning Goal
To understand what cell therapy is and what it is designed to do.
Cell therapy involves the transfer of live, “functional” cells into an individual to treat a disease. The cells may be autologous, meaning they originate from the person with the disease, or they may be allogeneic cells, which come from a donor.1
Cell Therapy1
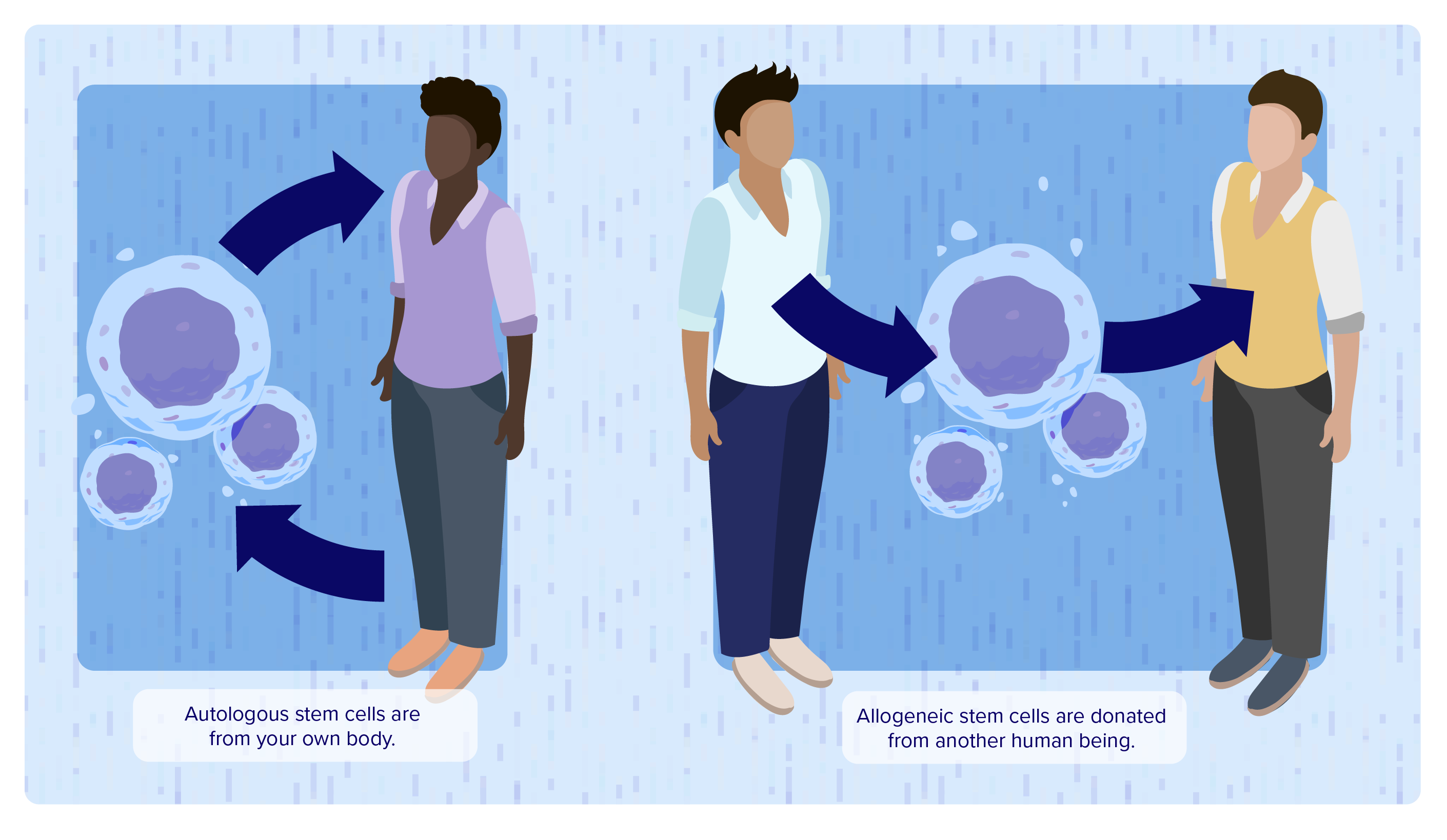
Autologous: cells from an individual are returned to the same individual
Allogeneic: cells from a donor are given to an individual
The cells used in cell therapy can be categorized by their potential to differentiate or develop into different cell types.2
- Differentiated or primary cells are a fixed type, meaning they cannot develop into other cell types.
- Multipotent cells can develop into certain cell types, but not as many types as pluripotent cells.
- Pluripotent cells can develop into any cell type in the body.
The type of cells used in cell therapy depends on the goal of the therapy.2
- The most common type of cell therapy is blood transfusion, by which a person can receive red blood cells from a donor to help replenish these cells in the recipient.2
- Another common cell therapy makes use of hematopoietic stem and progenitor cells (HSPCs). HSPCs are multipotent blood stem cells that give rise to all types of blood cells. HSPC transplantation, more commonly known as HSCT, makes use of HSPCs to repopulate the bone marrow compartment where stem cells reside.2 This procedure has been performed for more than 60 years.3
- More advanced forms of cell therapy may involve the use of gene therapy to modify the immune cells or stem cells that are being transferred.2
Cell therapies can be divided into two main types of applications:
- One application is in tissue engineering, in which cells are combined with scaffolds (structures of artificial or natural materials on which tissue is grown) and biologically active molecules to make functional tissues that restore, maintain, or improve damaged tissues or whole organs. Skin and cartilage are examples of engineered tissues that have been approved by the U.S. Food and Drug Administration (FDA).4
- The other main type of application involves the use of cells alone, which are modified to attack disease-causing cells, or to transfer therapeutic genetic material into the DNA of cells (transduction) to restore functional protein production. A notable example is chimeric antigen receptor T-cell (CAR T-cell) therapy, which is used to treat certain blood cancers such as leukemia and lymphoma, and is being studied for the potential treatment of other types of cancer.5
Additional Interesting Fact
ClinicalTrials.gov is a database of clinical trials conducted around the world. It features a searchable database that allows individuals to find trials they may be able to participate in, or to learn about new interventions or treatments that are being investigated.6
Key Learnings
Cell therapy, the transfer of live, “functional” cells into an individual to treat a disease, may involve the use of autologous (originating from the person with the disease) or allogeneic (from a donor) cells.
The cells used in cell therapy may be classified as differentiated, multipotent, or pluripotent, depending on their potential to develop into different cell types.
Cells can be combined with scaffolds and biologically active molecules to engineer tissue such as skin or cartilage, or used alone so that they are modified to attack disease-causing cells, such as in leukemia and lymphoma, or to transduce with a therapeutic gene to restore production of functional protein.
Continue learning about cell therapy in the next section
Section 2
Overview of Stem Cells
To understand the different types of stem cells and how they are used in gene therapy.
References
- Glossary. American Society of Gene + Cell Therapy; 2022. https://asgct.org/education/more-resources/glossary. Accessed June, 20, 2022.
- Gene and cell therapy FAQs. American Society of Gene & Cell Therapy; 2021. https://www.asgct.org/education/more-resources/gene-and-cell-therapy-faqs. Accessed December 22, 2021.
- Thomas ED, Lochte HL, WC Lu, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257(11):491-496. [PubMed]
- Tissue engineering and regenerative medicine. U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Biomedical Imaging and Bioengineering; 2021. https://www.nibib.nih.gov/science-education/science-topics/tissue-engineering-and-regenerative-medicine. Accessed December 22, 2021.
- CAR T-cell therapy. NCI Dictionary of Cancer Terms. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2021. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/car-t-cell-therapy. Accessed December 22, 2021.
- ClinicalTrials.gov. U.S. Department of Health and Human Services, National Institutes of Health, U.S. National Library of Medicine; 2021. https://clinicaltrials.gov/. Accessed December 22, 2021.