04 Understanding HSC Gene Therapy
Section 3
What is Conditioning?
Learning Goal
To understand the purpose and intent of conditioning in lentiviral hematopoietic stem and progenitor cell gene therapy, and how the process works.
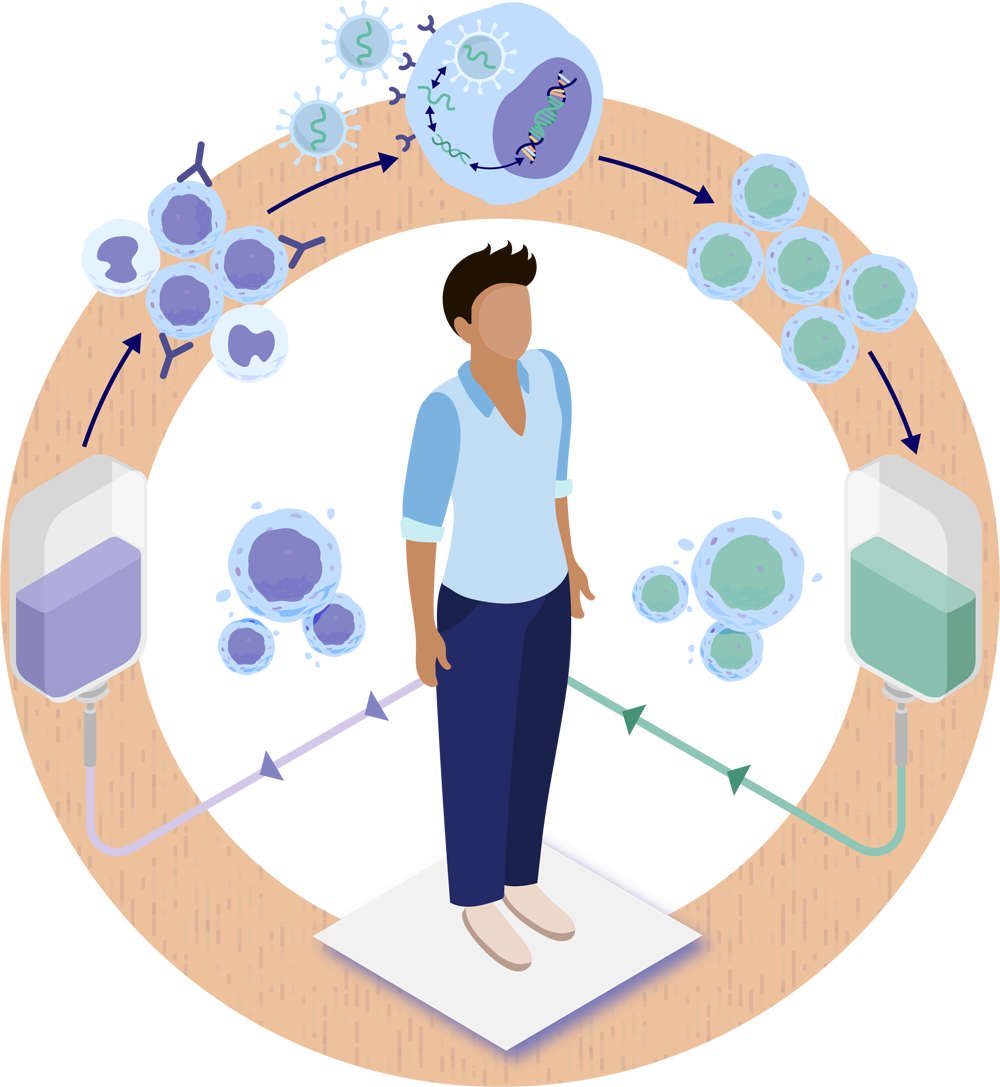
Conditioning is an important step in HSC gene therapy that prepares individuals’ bodies to receive back their own genetically modified stem cells. Conditioning involves the use of a conditioning agent to clear out some of the existing stem cells in order to make space in the bone marrow for the new, genetically modified stem cells. This is important because the newly created space allows the genetically modified stem cells to take hold (engraft) within the bone marrow, where they are expected to divide and produce generations of blood cells (also known as daughter cells) carrying the therapeutic gene. The goal is for the genetically modified stem cells to self-renew and divide to produce generations of daughter cells that are expected to carry the functional gene and make the functional protein that was missing.1-3
A conditioning agent is a medication that has been used as part of the group of medications for cancer treatment and stem cell transplants for many indications for several decades.4
- Although conditioning agents have been approved by regulatory agencies specifically for use in cancer treatment and stem cell transplants, none have been approved for use in HSC gene therapy. Conditioning agents are used in gene therapy clinical trials.5-9
The overall purpose of a chemotherapeutic agent is to kill cells. In the case of treating cancer, the goal of the agent is to kill the cancer cells and in doing so other non-cancerous cells also are killed. The goal of the agent in HSC gene therapy is to kill a portion of the cells in the bone marrow to allow space for the genetically modified stems cells to engraft in the bone marrow, a process referred to as conditioning.
After completion of conditioning, a person will receive back their genetically modified stem cells through an intravenous (IV) infusion. The genetically modified stem cells should begin to move into the space created in the bone marrow during conditioning.
Individuals should speak with a physician if they are considering participating in a clinical trial and have questions about the gene therapy or the conditioning process. Gene therapies are still under investigation, and the potential short-term and long-term side effects of gene therapy are not fully understood. A doctor can also advise on the potential management of side effects that may be experienced during the gene therapy process.
Key Learnings
Conditioning involves the use of a conditioning agent to clear out some of the existing stem cells in the bone marrow in order to make space for new, genetically modified stem cells.
A conditioning agent is a medication that has been used as part of the group of medications for cancer treatment and stem cell transplants for many decades.
In cancer treatment, the purpose of chemotherapy is to eradicate all the cancer cells. The medication is used in combination with other agents, over multiple cycles.
In HSC gene therapy, a single conditioning agent is typically used in a single cycle to make space in the bone marrow.
Continue learning about HSC gene therapy in the next section
Section 4
What is the HSC Gene Therapy Process?
To understand the experience of undergoing HSC gene therapy.
References
- Drysdale CM, Tisdale JF, Uchida N. Immunoresponse to gene-modified hematopoietic stem cells. Mol Ther Methods Clin Dev. 2020;16:42-49. [PubMed]
- Piguet F, Alves S, Cartier N. Clinical gene therapy for neurodegenerative diseases: past, present, and future. Hum Gene Ther. 2017;28(11):988-1003. [PubMed]
- Capotondo A, Milazzo R, Politi LS, et al. Brain conditioning is instrumental for successful microglia reconstitution following hematopoietic stem cell transplantation. Proc Natl Acad Sci U S A. 2012;109(37):15018-15023. [PubMed]
- Busulfan Injection prescribing information. Sagent Pharmaceuticals, Inc.; 2020.
- Sessa M, Lorioli L, Fumagalli F, et al. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: an ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet. 2016;388:476-487. [PubMed]
- Walters MC, Locatelli F, Thrasher EJ, et al. Safety of autologous hematopoietic stem cell transplantation with gene addition therapy for transfusion-dependent β-Thalassemia, sickle cell disease, and cerebral adrenoleukodystrophy. Biol Blood Marrow Transplant. 2020;26(3 Suppl):S38-S39. doi: https://doi.org/10.1016/j.bbmt.2019.12.104.
- Thomas M, Nicholls K, Carnley B, et al. AVR-RD-01, an investigational lentiviral gene therapy for Fabry disease: overview of clinical data from phase 1 and phase 2 studies. Mol Genet Metab. 2021;132(2):S106. https://doi.org/10.1016/j.ymgme.2020.12.259.
- Jacobsen L, Kerner JA, Ciotti R, et al. The GuardOne clinical trial: a first-in-human, open label, multinational phase 1/2 study of AVR-RD-02 ex vivo lentiviral vector, autologous gene therapy for Gaucher disease. Mol Genet Metab. 2021;132(2):S51-S52. https://doi.org/10.1016/j.ymgme.2020.12.112.
- Cherqui S. Hematopoietic stem cell gene therapy for cystinosis: updated results from a phase I/II clinical trial. Oral presentation at: WORLDSymposium; February 12, 2021.