04 Understanding HSC Gene Therapy
Section 4
What is the HSC Gene Therapy Process?
Learning Goal
To understand the experience of undergoing HSC gene therapy.
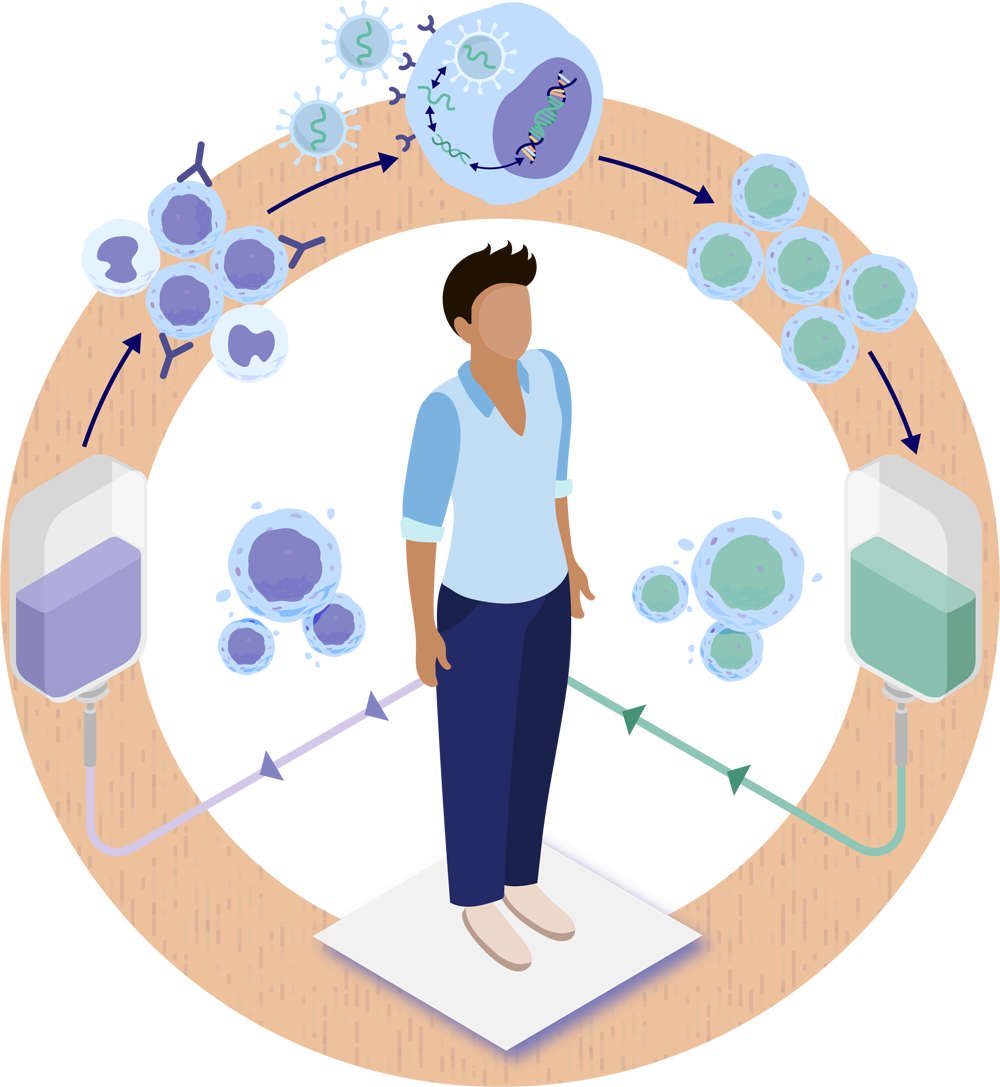
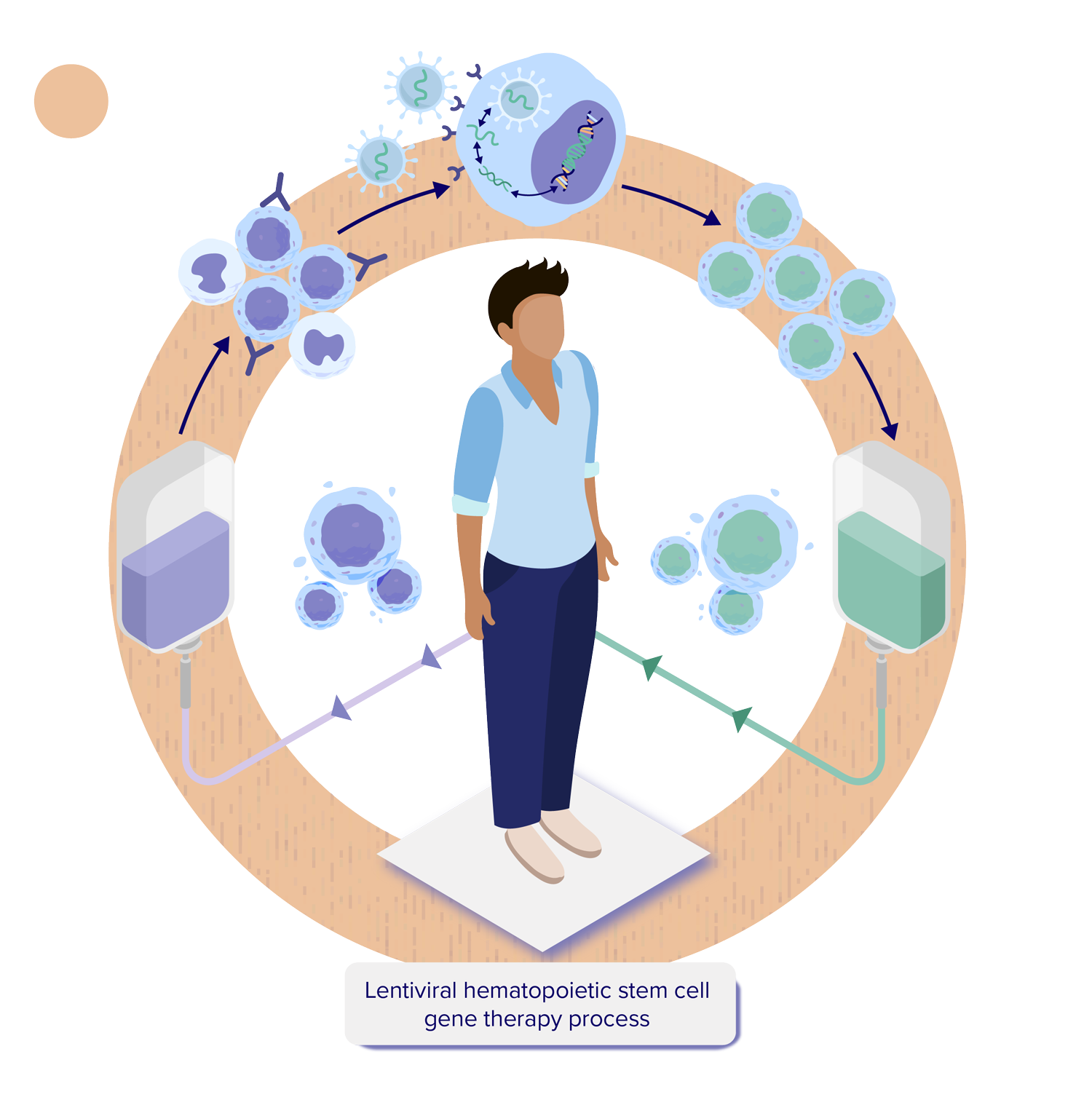
For a person undergoing HSC gene therapy, the process begins with collecting hematopoietic stem and progenitor cells (HSPCs) from the person’s blood. As shown in the illustration, a lentiviral vector delivers therapeutic genetic material into the person’s own HSPCs. These vectors are expected to integrate the therapeutic gene directly into the DNA of the HSPCs. The genetically modified stem cells are returned to the body via an intravenous (IV) infusion, and then settle back into the bone marrow. The cells will then resume their job of producing the different types of blood cells circulating throughout the body, as well as immune cells in the brain, but this time they are expected to carry the therapeutic gene.1-4
Here we provide more detail about the steps involved in the treatment journey for a person undergoing HSC gene therapy4-9:
 1
2
3
4
5
6
1
2
3
4
5
6 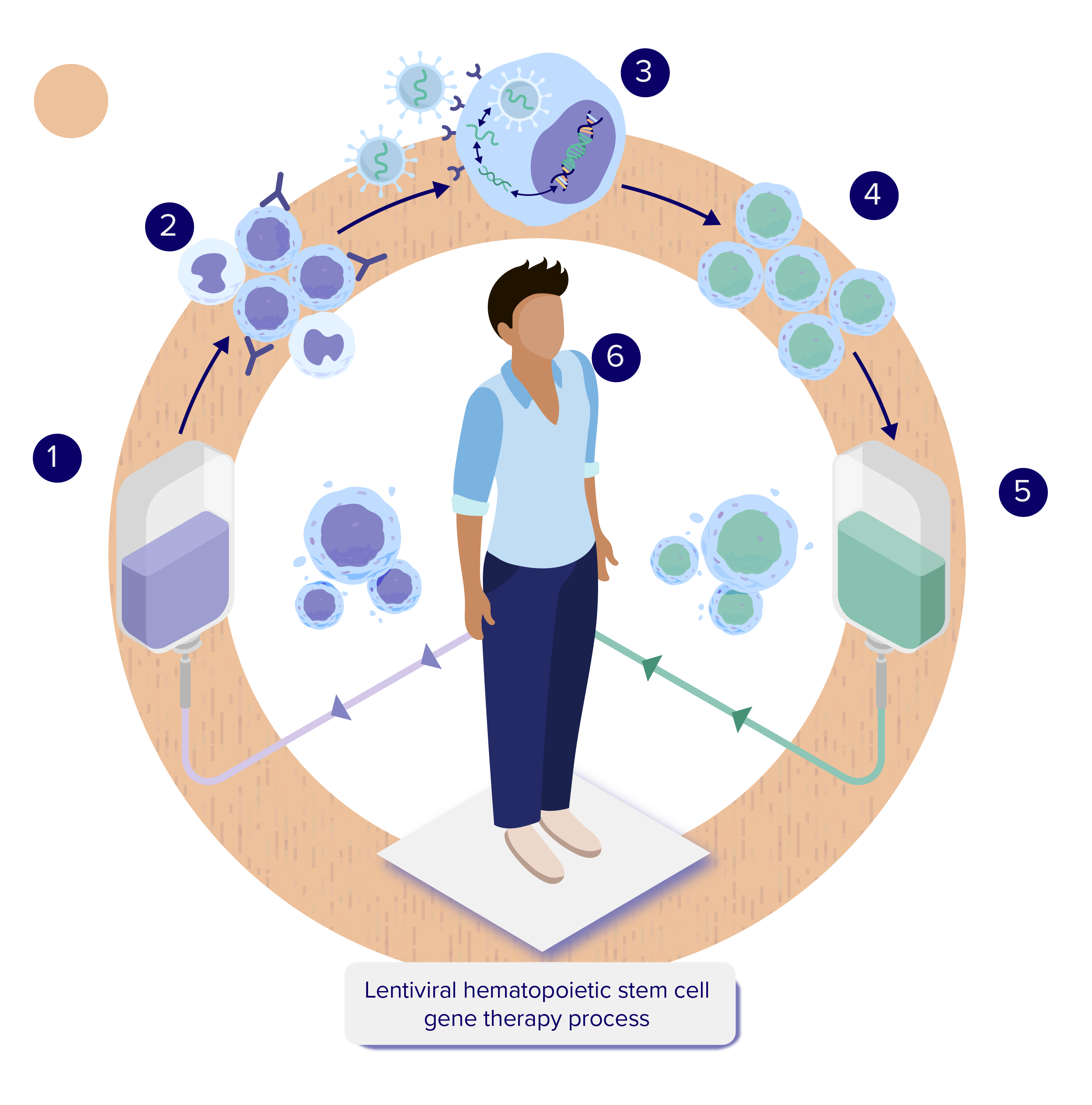
- The person receives a combination of medications designed to mobilize or release their HSPCs to move out of the bone marrow and into the blood. The HSPCs are collected through a process called apheresis or via bone marrow aspiration and then transported to a regional manufacturing site.4-8 See Overview of Stem Cells for additional information.
- In the manufacturing facility, HSPCs expressing the cell surface marker CD34 are isolated and separated out. These undifferentiated HSPCs, known as CD34+ cells, will produce all the different types of blood cells.4-8 See Overview of Hematopoietic Stem Cells (HSCs) for additional information.
- The HSPCs are combined with a lentiviral vector, which delivers the therapeutic genetic material into the HSPCs (transduction).4-8 See How is Gene Therapy Delivered? for additional information.
- The HSPCs with the new therapeutic gene (genetically modified stem cells) are then collected and frozen.4-8 See How is Hematopoietic Stem Cell Gene Therapy Made? for additional information.
- The person goes through a conditioning process to prepare their body to receive back their own genetically modified stem cells by creating space in the bone marrow.4-8 See What is Conditioning? for additional information.
- After the cells are thawed, they are administered using an intravenous (IV) infusion. Once in the body, the genetically modified stem cells containing the therapeutic gene should begin to move into the space created in the bone marrow during conditioning. Some genetically modified HSPCs may form specialized daughter cells called monocytes which can enter the brain, where they may become microglial cells that are essential to the function of the central nervous system (CNS, consisting of the brain and spinal cord). The genetically modified stem cells should then divide asymmetrically to form more stem cells, as well as different types of blood cells, and are expected to make the functioning protein such as the enzyme that was previously missing or not working.9
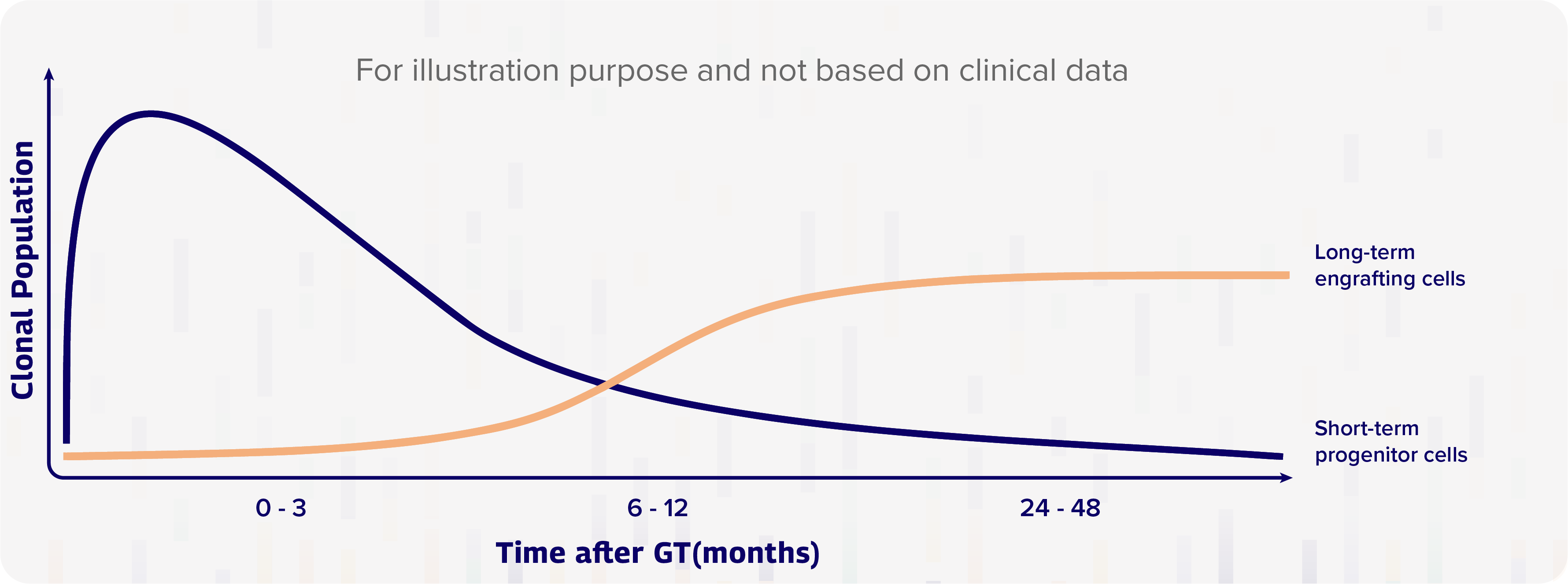
Research suggests that the transplanted genetically engineered CD34+ cells (genetically modified stem cells) act in two distinct “waves”: the first wave, which takes place immediately within the first 6-12 months, principally involves the rapid division of progenitor cells, which are early descendants of hematopoietic stem cells (HSCs) that can form in one or more cell type, but cannot divide and reproduce indefinitely; in the second wave, a smaller quantity of long-term engrafting HSCs start to take over 6-12 months post-therapy – just as the effects of the first wave start to decline; these cells, which engraft in the bone marrow and start to produce significant numbers of daughter cells, will last for the rest of the person’s life.3 The following diagram helps to illustrate the potential for HSC gene therapy to provide long-lasting production of a therapeutic protein.
Gene therapy consists of progenitor (short lived) cells and long-term engrafting cells3
Key Learnings
The HSC gene therapy process begins with the collection of HSPCs from the person’s blood, after which a lentiviral vector delivers therapeutic genetic material into the HSPCs.
After undergoing conditioning, the person receives the genetically modified stem cells via an intravenous (IV) infusion. The cells then settle back into the bone marrow as well as in the brain, where they are expected to divide to form more stem cells and produce different types of blood cells while carrying the therapeutic gene.
The goal is that newly produced blood cells make the functioning protein that was previously missing or not working.
Once infused, there are two distinct “waves” of cell formation:
- The first wave involves rapid division of progenitor cells within the first 6-12 months.
- The second wave is produced by a smaller quantity of long-term engrafting hematopoietic stem cells (HSCs) which engraft in the bone marrow and start to produce significant numbers of daughter cells at 6-12 months post-therapy.
- Some genetically modified HSPCs form specialized daughter cells called monocytes, which engraft in the CNS as microglial cells.
Continue learning in the next section
Topic 5: Section 1
Online Resources
Looking for more great information about gene and cell therapy? Check out these online resources.
References
- Milone MC, O’Doherty U. Clinical use of lentiviral vectors. Leukemia. 2018;32:1529. [PubMed]
- What Is Gene Therapy? How Does It Work? U.S. Food & Drug Administration; 2017. https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm589197.htm. Accessed December 23, 2021.
- Biasco L, Pellin D, Scala S, et al. In vivo tracking of human hematopoiesis reveals patterns of clonal dynamics during early and steady-state reconstitution phases. Cell Stem Cell. 2016;19:107-119. [PubMed]
- Staal FJT, Aiuti A, Cavazzana M. Autologous stem-cell-based gene therapy for inherited disorders: state of the art and perspectives. Front Pediatr. 2019;7:443. [PubMed]
- Naldini L. Genetic engineering of hematopoiesis: current stage of clinical translation and future perspectives. EMBO Mol Med. 2019;11:e9958. [PubMed]
- Morgan RA, Gray D, Lomova A, Kohn DB. Hematopoietic stem cell gene therapy: progress and lessons- learned. Cell Stem Cell. 2017;21(5):574-590. [PubMed]
- Drysdale CM, Tisdale JF, Uchida N. Immunoresponse to gene-modified hematopoietic stem cells. Mol Ther Methods Clin Dev. 2020;16:42-49. [PubMed]
- Piguet F, Alves S, Cartier N. Clinical gene therapy for neurodegenerative diseases: past, present, and future. Hum Gene Ther. 2017;28(11):988-1003. [PubMed]
- Wolf NI, Breur M, Plug B, et al. Metachromatic leukodystrophy and transplantation: remyelination, no cross-correction. Ann Clin Transl Neurol. 2020;7(2):169-180. [PubMed]